Global Chemical Management & Product Excellence
Management Approach: Product Stewardship
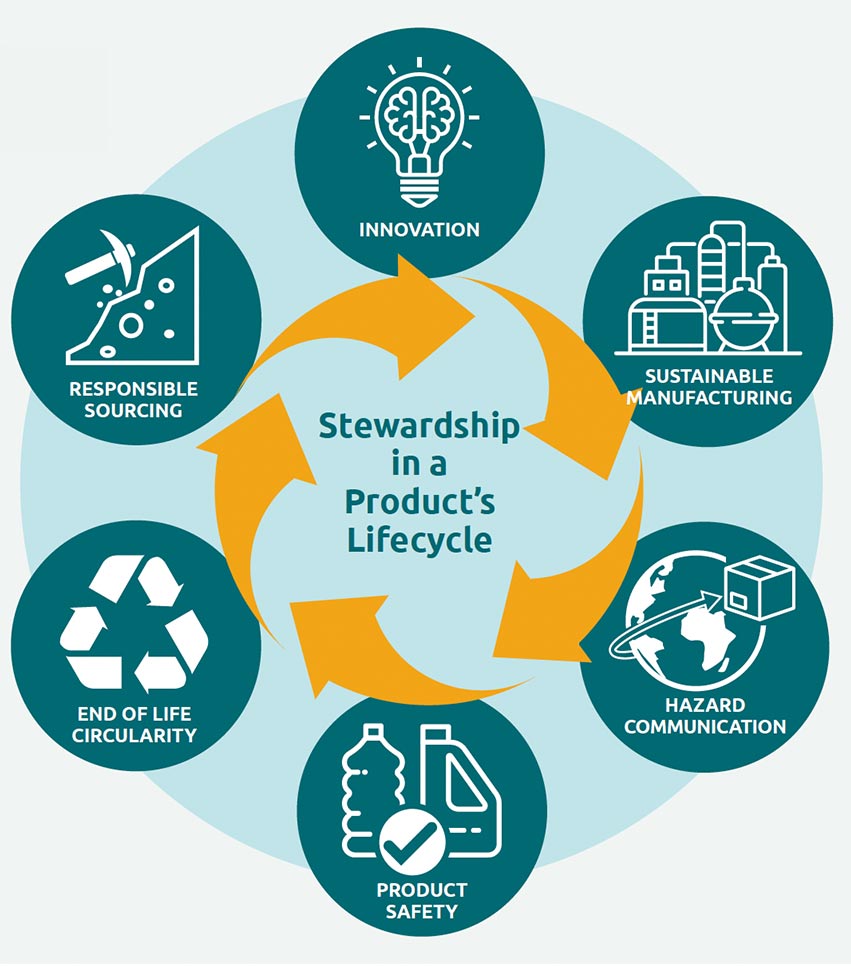
Protecting associates, customers and the environment by providing safe products is a priority for Avient. We review the health, safety and environmental impact of our products throughout all life cycle stages, from responsible sourcing of our raw materials through product development and manufacturing to end applications at the customer and beyond.
Product Stewardship supports the Avient portfolio by providing information related to the safe use and handling of products through hazard communication, and by complying with global and local regulations. Continuous customer communications on product safety related to new and existing applications are an integral part of understanding the health and safety impacts of our product portfolio.
We maintain our certification to the American Chemistry Council’s (ACC) Responsible Care® management system and comply with their Product Safety Code which goes beyond basic legal requirements. For 2023, there have been zero incidences of non-compliance with both legally required and voluntary standards, and there have been no incidences of our products harming people or the environment. In addition, for this reporting period there have been zero incidences of product recalls due to non-compliance in the markets we serve.

Strategy
Avient is actively involved in various associations including the ACC, European MasterBatchers and Compounders (EuMBC), European Plastics Converters (EuPC) and Titanium Dioxide Manufacturers Association (TDMA). Industry best practices and efforts to minimize impacts of our products on human health and the environment are based on a combination of lessons learned through these outreach programs and compliance.
Product Stewardship uses the ACC prioritization tool and associated risk assessment methodology to identify, document and communicate environmental, health and safety impacts of our products. Five percent or less of our products by total sales revenue are classified as category 1 or 2 health hazards in accordance with the Globally Harmonized system (GHS) for classification. These classifications and others, such as, environmental classifications (Persistent, Bioaccumulative Toxins—PBTs) and physical form contribute to the overall prioritization. Substances are encapsulated in our solid polymers and not biologically available for exposure when used as intended, therefore do not present a hazard. The majority of our products are not labeled or transported as hazardous materials. Despite this, we are committed to the process and have completed 43% of prioritized risk assessments. The number of prioritized risk assessments continue to grow each year as the process matures. Our goal is to complete 100% of risk assessments on hazardous materials greater than 1 ton by 2027.
As a result of these assessments, we have published additional substance safety summaries on our Avient website and will continue to update internal and external stakeholders on material handling and environmental considerations. We maintain data related to the environmental, health and safety of our products in our product stewardship database. The information is reviewed and updated continuously. Through this data, we create safety data sheets and customer certification statements for intended use and product safety improvements.
Global Chemical Management
The current and future focus is on continued Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) assessments, reclassifications and communication on the safe use and handling of these substances. Many other countries are following suit on the EU REACH chemicals regulation or a similar framework. We continuously monitor these global emerging regulations and the impact to our product portfolio.
As part of the management and monitoring of Global Chemical Control Laws, Product Stewardship continuously monitors regulatory lists that identify hazardous substances of concern. Product Stewardship compares the substance lists published by the authorities to our product portfolio. This enables us to identify any substances coming under review as soon as possible and take appropriate action. This includes working with suppliers and other departments within Avient to identify suitable alternatives and drive innovation towards sustainable solutions. Such lists include REACH Substances of Very High Concern (SVHC) candidate list, U.S. Toxic Substances Control Act (TSCA), International Agency for Research on Cancer (IARC) and California Prop 65 amongst others.
We continue to update Poison Center notifications associated with our European portfolio and monitor risk assessments associated with the U.S. Environmental Protection Agency’s (EPA) Toxic Substance Control Act (TSCA) inventory reset rule. We continue to actively monitor the developing regulatory activities at state, national and global levels with respect to per and polyfluoroalkyl substances (PFAS). PFAS covers thousands of different substances and definitions of PFAS differ across organizations, states, and countries. Under the broad definition, PFAS includes fluoropolymers which are large, stable, inert polymeric molecules. Polymeric, high molecular weight fluoropolymers are too large to cross biological membranes. Avient does currently purchase and use a small amount of fluoropolymers and PFAS containing additives as raw materials which represent less than 3% of our global product portfolio. Avient does not use perfluorooctanoic acid (PFOA) or perfluorooctane sulfonic acid (PFOS) in its product formulations.
Management and monitoring of global inventory regulations and other emerging issues by our regional product stewardship experts ensures a quick evaluation and proactive response to potential regulatory risks. Avient’s commitment to managing and monitoring the impact of global regulatory requirements on our businesses is demonstrated through our Global Chemical Management Policy.
Digitization Strategy
The global regulatory environment of today is more complex than ever before. Avient is committed to having expert systems in place to manage complex, global regulatory compliance requirements and mitigate potential risk.
In 2023, Avient invested in a new product stewardship platform to manage the environmental, health and safety impact of the Avient portfolio, the development and implementation of this platform will continue as we move into 2024. Through this powerful, configurable and global software for the management of regulatory and voluntary standard compliance, we will have full data transparency throughout the life cycle of Avient products. This investment will improve customer experience and it reiterates our commitment to providing transparency and communication with our stakeholders regarding our products.

Product Excellence
Management Systems
Avient subscribes to several third party management systems across the globe, including ISO and Responsible Care to ensure continual improvement. External third-party certification is an important part of ensuring our products are both safe and produced in alignment with industry standard best practices. To find more details about our certifications, visit our ISO Certifications Library.
Avient has 102 global manufacturing facilities. 96% of these sites are certified through independent third parties to management system standards including ISO:14001, ISO:50001, ISO:22000, ISO:45001, ISO:9001, and Responsible Care®. Avient successfully achieved a Global Central Certification for RC14001 Management System for 48 locations. The certification allows all locations on the certification to maintain a Responsible Care® Certification and ISO:14001 certification. In addition to our global certifications, individual sites have obtained additional certification specific to the products and customers they serve. These certifications include ISCC Plus, Global Recycled Standard (GRS) 4.0, bluesign® SYSTEM, and others. International standards cover areas of Quality, Environmental, Health and Safety, Automotive, Medical Device, Responsible Care® and Energy Management.
For this reporting period, there have been no major non-conformances associated with management system certifications of Avient facilities. There have been zero product recalls associated with the quality of Avient products. For our sensitive applications we have implemented Good Manufacturing Processes (GMP) and ISO 13485. 100% of our facilities producing for the healthcare market are GMP or ISO audited within a 3–year period. There were no significant audit findings preventing manufacturing in this reporting period.
Product Carbon Footprint
Avient recognizes that given our location in the supply chain we are well positioned to enable sustainability along the value chain, through innovation at the early to mid-stages of the product life cycle. For Avient, the most material aspect of Life Cycle Analysis (LCA) is our Product Carbon Footprint (PCF).
In 2022, we established a methodology to standardize our approach to calculating PCF. This methodology was developed in accordance with ISO 14067:2018 for product carbon footprint and is aligned with ISO 14040/140440 for life cycle analysis. We have received third party certification from TÜV Rheinland. We continue to partner with Carbon Minds and well as our supply chain to generate the data. To date we have completed over 1,000 product carbon footprint calculations. Our goal in 2023 is to fully automate this process and expand the PCF data available for the Avient portfolio.

ZERO
Major Non-Conformances at ISO Sites
96 %
Manufacturing Sites Certified to an International Standard
1,500+
Product Carbon Footprint Calculations